New Paper in Angewandte Chemie by the Piel Lab
Modular Halogenation, α-Hydroxylation, and Acylation by a Remarkably Versatile Polyketide Synthase
Franziska Hemmerling, Roy A Meoded, Amy Fraley, Hannah Minas, Cora Dieterich, Michael Rust, Reiko Ueoka, Katja Jensen, Eric Helfrich, Cedric Bergande, Maurice Biedermann, Nancy Magnus, Birgit Piechulla, Jörn Piel
Angew Chem Int Ed Engl. (2022) Jan 12.
doi: 10.1002/anie.202116614.
Bacterial multimodular polyketide synthases (PKSs) are large enzymatic assembly lines that synthesize many bioactive natural products of therapeutic relevance. While PKS catalysis is mostly based on fatty acid biosynthetic principles, polyketides can be further diversified by post-PKS enzymes.
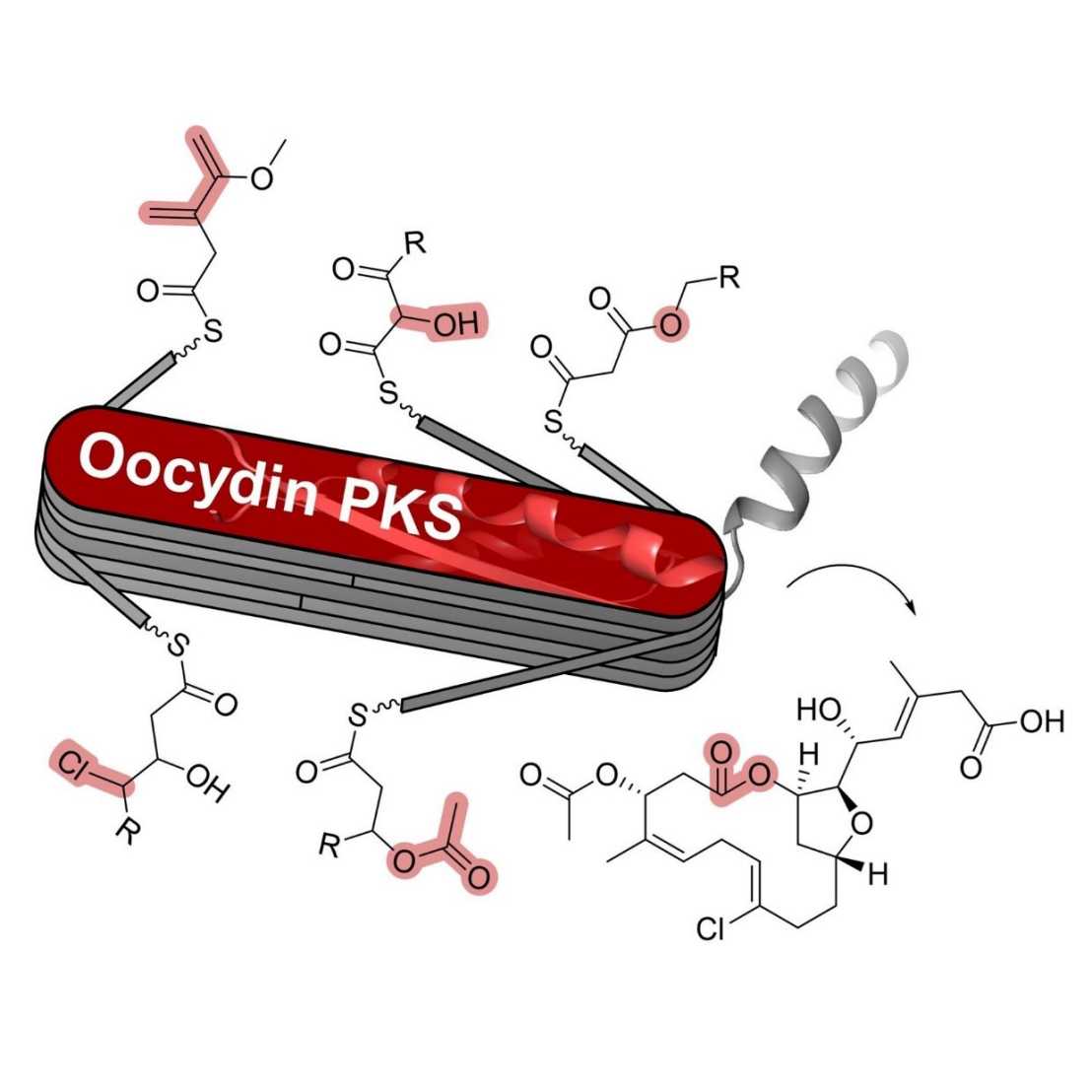
Here, we characterized a remarkably versatile trans-acyltransferase (trans-AT) PKS from Serratia that builds structurally complex macrolides via more than ten functionally distinct PKS modules. In the oocydin PKS, we identified a new oxygenation module that α-hydroxylates polyketide intermediates, a halogenating module catalyzing backbone γ-chlorination, and modular O-acetylation by a thioesterase-like domain. These results from a single biosynthetic assembly line highlight the expansive biochemical repertoire of trans-AT PKSs and provide diverse modular tools for engineered biosynthesis from a close relative of E. coli.
Link to the D BIOL news article
Link to the paper in external page AngewandteChemie International Edition