New PNAS Nexus Paper by the Piel Lab
Single-cell metabolite detection and genomics reveals uncultivated talented producer
Masato Kogawa, Rimi Miyaoka, Franziska Hemmerling, Masahiro Ando, Kei Yura, Keigo Ide, Yohei Nishikawa, Masahito Hosokawa, Yuji Ise, Jackson K B Cahn, Kentaro Takada, Shigeki Matsunaga, Tetsushi Mori, Jörn Piel, Haruko Takeyama
PNAS Nexus, Volume 1, Issue 1, March (2022): pgab007
https://doi.org/10.1093/pnasnexus/pgab007
In a recent PNAS Nexus paper, the Piel group (IMB) in collaboration with groups from Waseda University and the University of Tokyo used a Raman microscopy-single-bacterial genomics approach to reveal a chemically rich bacterial symbiont in the complex microbiome of a marine sponge.
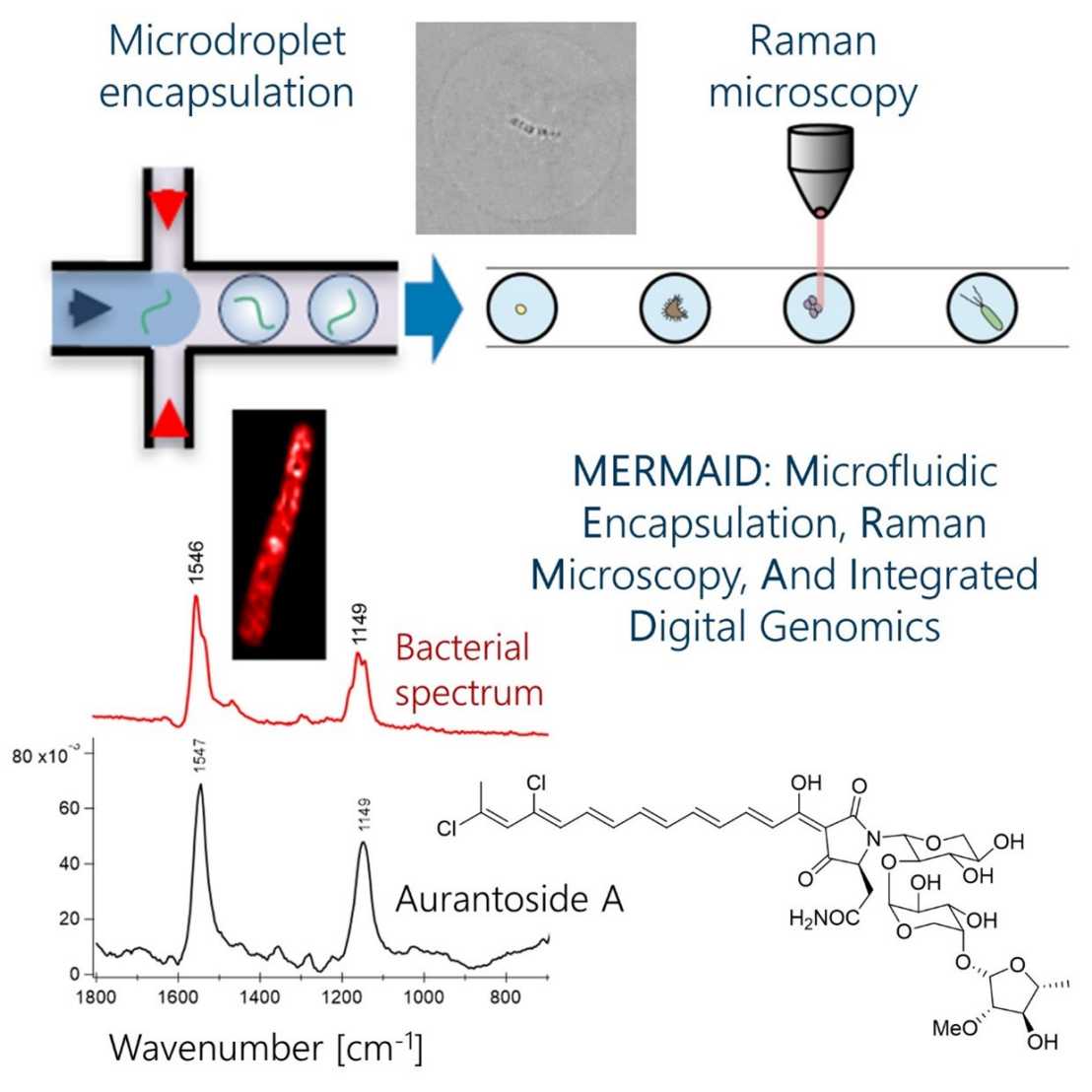
The production of bioactive metabolites is increasingly recognized as an important function of host-associated bacteria. An example is defensive symbiosis that might account for much of the chemical richness of marine invertebrates including sponges (Porifera), one of the oldest metazoans. However, most bacterial members of sponge microbiomes have not been cultivated or sequenced, and therefore, remain unrecognized. Unequivocally linking metabolic functions to a cellular source in sponge microbiomes is, therefore, a challenge. Here, we report an analysis pipeline of microfluidic encapsulation, Raman microscopy, and integrated digital genomics (MERMAID) for an efficient identification of uncultivated producers. We applied this method to the chemically rich bacteriosponge (sponge that hosts a rich bacterial community) Theonella swinhoei, previously shown to contain ‘Entotheonella’ symbionts that produce most of the bioactive substances isolated from the sponge. As an exception, the antifungal aurantosides had remained unassigned to a source. Raman-guided single-bacterial analysis and sequencing revealed a cryptic, distinct multiproducer, Candidatus 'Poriflexus aureus’ from a new Chloroflexi lineage as the aurantoside producer. Its exceptionally large genome contains numerous biosynthetic loci and suggested an even higher chemical richness of this sponge than previously appreciated. This study highlights the importance of complementary technologies to uncover microbiome functions, reveals remarkable parallels between distantly related symbionts of the same host, and adds functional support for diverse chemically prolific lineages being present in microbial dark matter.
Link to the publication in external page PNAS Nexus