New Paper in Angewandte Chemie by the Piel and the Oxenius Labs
Aquimarins, Peptide Antibiotics with Amino‐Modified C‐Termini from a Sponge‐Derived Aquimarina sp. Bacterium
Cora L. Dieterich, Silke I. Probst, Reiko Ueoka, Ioana Sandu, Daniel Schäfle, Michael Dal Molin, Hannah A. Minas, Rodrigo Costa, Annette Oxenius, Peter Sander, Jörn Piel
Angew. Chem. Int. Ed. (2021), e202115802
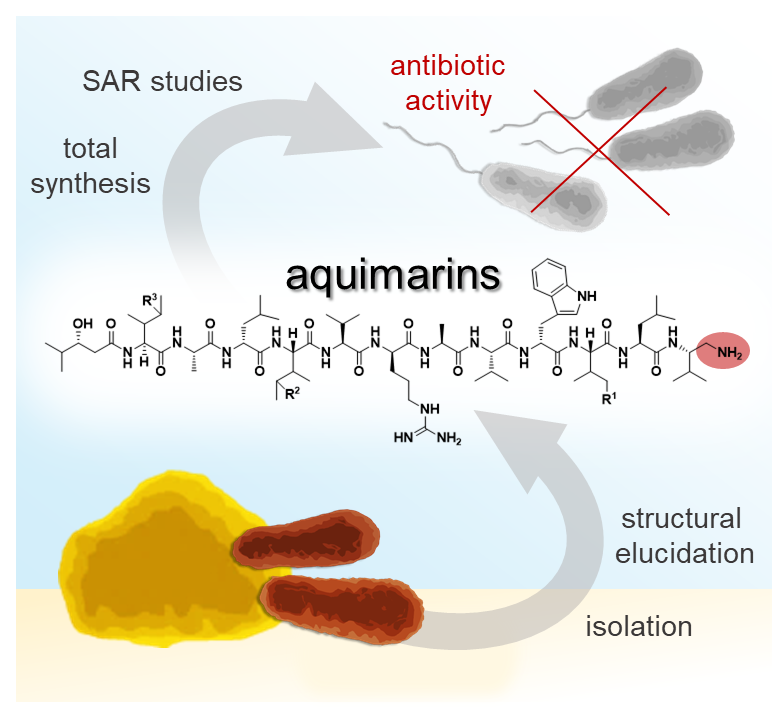
Genome mining and bioactivity studies suggested the sponge-derived bacterium Aquimarina sp. Aq135 as a producer of new antibiotics. Activity-guided isolation identified antibacterial peptides, named aquimarins, featuring a new scaffold with an unusual C-terminal amino group and chlorine moieties. Responsible for the halogenation is the Fe(II)/α-ketoglutarate-dependent chlorinase AqmA that halogenates up to two isoleucine residues in a carrier protein-dependent fashion. Total syntheses of two natural aquimarins and eight non-natural variants were developed. Structure-activity relationship (SAR) studies with these compounds showed that the synthetically more laborious chlorinations are not required for antibacterial activity but enhance cytotoxicity. In contrast, variants lacking the C-terminal amine were virtually inactive, suggesting diamines similar to the terminal aquimarine residue as candidate building blocks for new peptidomimetic antibiotics.
Link to the D-BIOL News Article.
Link to the Paper in external page Angewandte Chemie