New Paper in Angew. Chem. Int. Ed. by the Piel Lab
Posttranslational arginases provide a ribosomal route to non‐proteinogenic ornithine residues in diverse peptide sequences
Silja Mordhorst, Brandon I. Morinaka, Anna L. Vagstad and Jörn Piel
Angew. Chem. Int. Ed. (2020) Accepted Author Manuscript. doi:10.1002/anie.202008990
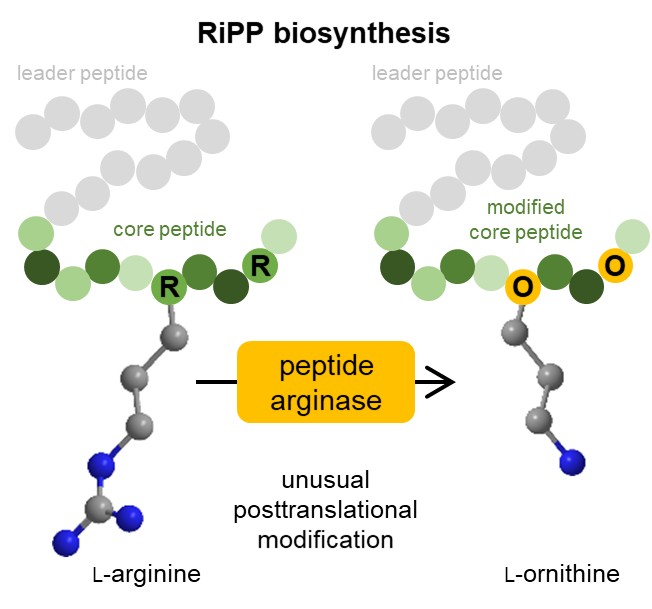
Ornithine is a component of many bioactive nonribosomal peptides but has been challenging to incorporate into ribosomal products. We recently identified OspR, a cyanobacterial arginase‐like enzyme that installs ornithines in the antiviral ribosomally synthesised and posttranslationally modified peptide (RiPP) landornamide A. Here we report that OspR belongs to a larger family of peptide arginases from diverse organisms and RiPP types. In E. coli expressions, seven selected enzymes converted arginine residues to ornithines with little preference for the leader type. A diverse range of peptide sequences was modified, including polyarginine repeats. Further exploring the synthetic potential of OspR, we generated analogues of ornithine‐containing nonribosomal peptides using RiPP technology. Five pseudo‐nonribosomal products with ornithines at correct positions were obtained. This included a brevicidine analogue containing ornithine and a d‐amino acid that was installed by the peptide epimerase OspD, suggesting new opportunities for peptide bioengineering.