Joint Angewandte Chemie paper by the Piel and the Oxenius labs
Landornamides, antiviral ornithine‐containing ribosomal peptides discovered by proteusin mining
Nina Bösch, Mariana Borsa, Ute Greczmiel, Brandon Morinaka, Muriel Gugger, Annette Oxenius, Anna Lisa Vagstad, Jörn Piel
Angew Chem Int Ed Engl. 2020 Mar 12. doi: 10.1002/anie.201916321.
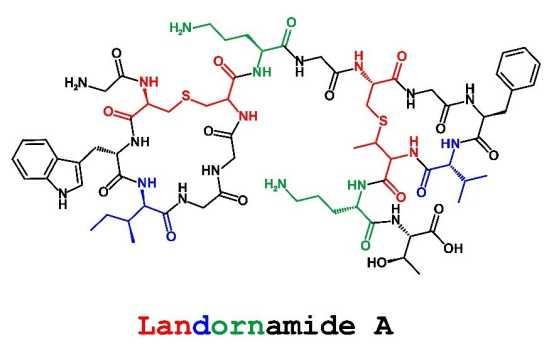
Proteusins are a family of bacterial ribosomal peptides that largely remain hypothetical, genome-predicted metabolites. The only known members are the polytheonamide-type cytotoxins with remarkably complex structures due to numerous unusual posttranslational modifications (PTMs). Cyanobacteria contain large numbers of putative proteusin loci with highly variable sets of PTM gene candidates. Interrogating whether this gene diversity offers chemical and pharmacological discovery potential beyond polytheonamide-type compounds, we characterized landornamide A, the product of the silent osp gene cluster from Kamptonema sp. PCC 6506. Pathway reconstruction in E. coli revealed a peptide combining lanthionines, d-residues, and, as a novel PTM, two ornithines introduced by the arginase-like enzyme OspR. Landornamide A inhibited lymphocytic choriomeningitis virus infecting mouse fibrosarcoma cells, representing one of the few known anti-arenaviral compounds. The data support proteusins as a rich resource of chemical scaffolds, new maturation enzymes, and bioactivities.
Link to the ETH D BIOL article
Link to the paper in external page Angewandte Chemie - International Edition