Project 4: Biosynthesis and characterization of fungal RiPPs
Peptide backbone N-methylations and macrocyclization are desired features of peptide therapeutics, as these modifications can improve cell permeability, target selectivity, and proteolytic stability. One famous example of a backbone N-methylated peptide macrocycle used as a therapeutic is the immunosuppressant cyclosporin A, a fungal non-ribosomal peptide natural product.
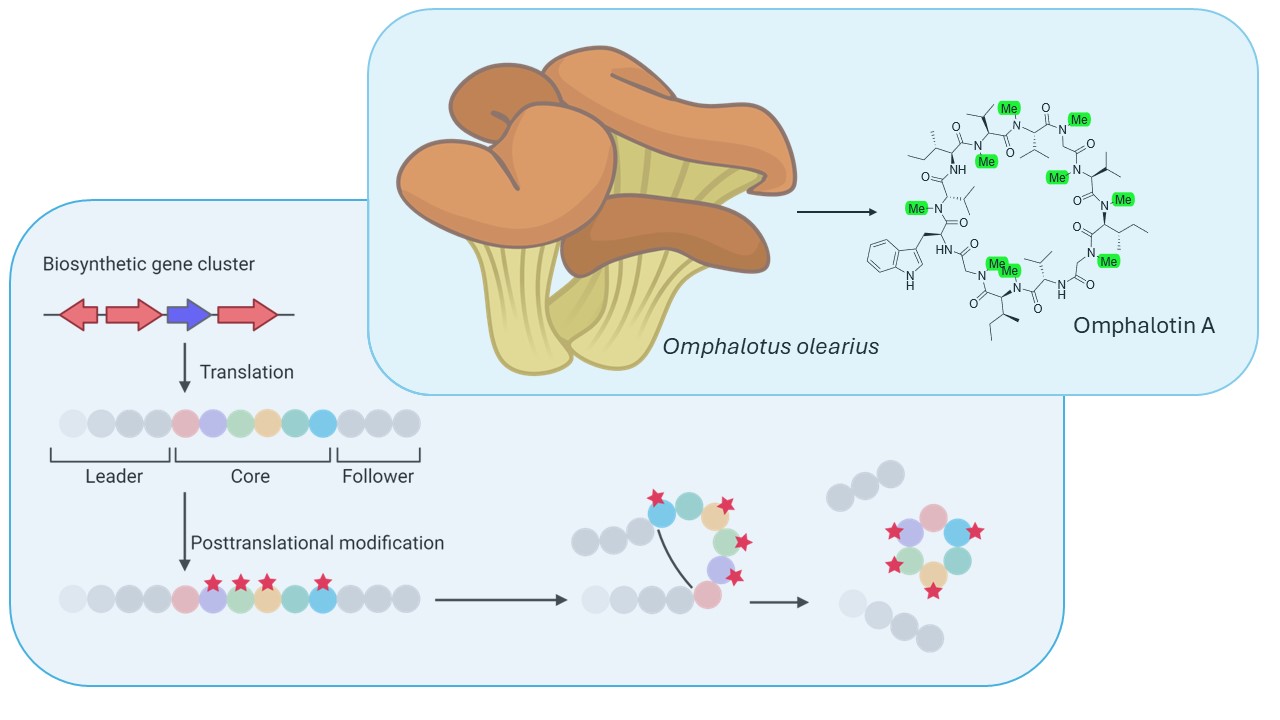
Another fungal peptide natural product with structural similarity to cyclosporin A is omphalotin A. This peptide macrocycle is a ribosomally produced and post-translationally modified peptide (RiPP) that consists of 12 amino acids, 9 of which are backbone N-methylated. This compound, produced by the mushroom Omphalotus olearius, exhibits strong toxicity against nematodes by an unknown mechanism.
We investigate the biosynthetic pathway of omphalotin A as a potential biotechnological platform to produce multiply backbone N-methylated peptide macrocycles. We established the heterologous production of omphalotin A, which will enable the biosynthesis of novel backbone N-methylated peptide macrocycles with potentially promising pharmacological properties. In addition, the efficient bacterial production of omphalotin A will allow us to screen for the still unknown molecular target of omphalotin A.